WHY THEY'RE IMPORTANT AND HOW PH AFFECTS THEIR BIOAVAILABILITY IN SOILWhen it comes to making the most of trace elements in the soil, plants are incredibly resourceful! It’s no secret that carbon (C), oxygen (O) and hydrogen (H) are the building blocks of life. Lipids (fats) and sugars are made of C, H & O. These molecules provide energy in different forms; not just for plants, but for all forms of life. All organisms are also made up of cells. Each cell is surrounded by a cell membrane, which is made of lipids. Fortunately, C, H & O are more than abundant in most environments (our atmosphere is loaded with CO2, O2, H2 and H20). In plant science, C, H & O are referred to as Basic Nutrients.
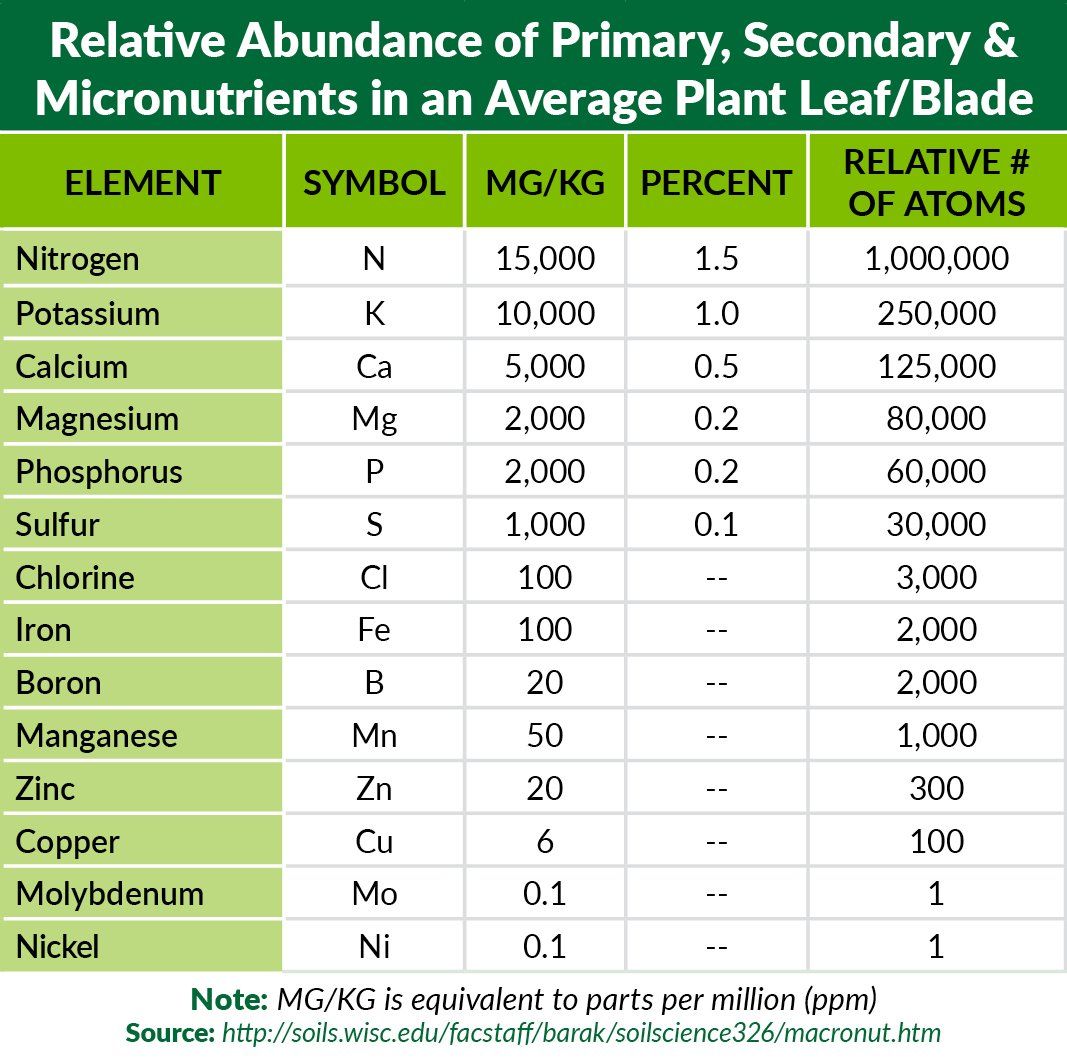
We are all more than familiar with the Primary Nutrients as well;
Nitrogen,
Phosphorous, and
Potassium
(N-P-K). Healthy turf metabolism depends on an ample supply of these three elements. When any of these three (particularly nitrogen) are depleted in the soil, plant growth visibly suffers.
Also of great importance is the Secondary Nutrients: Calcium, Magnesium and Sulfur. Calcium plays an important role in cell signaling, cell division mechanisms, and disease resistance. Magnesium is of paramount importance to plants; every single chlorophyll molecule has a magnesium ion located at its center. Without magnesium, photosynthesis could not take place. Sulfur is present in many amino acids and proteins. Even though it is not needed in large quantities in northern regions, sulfur also acts as a soil conditioner by helping to mitigate sodium uptake in plants (sodium is usually in excess in most environments and is considered an abiotic stressor).
MICRONUTRIENT ROLESIron (Fe) –
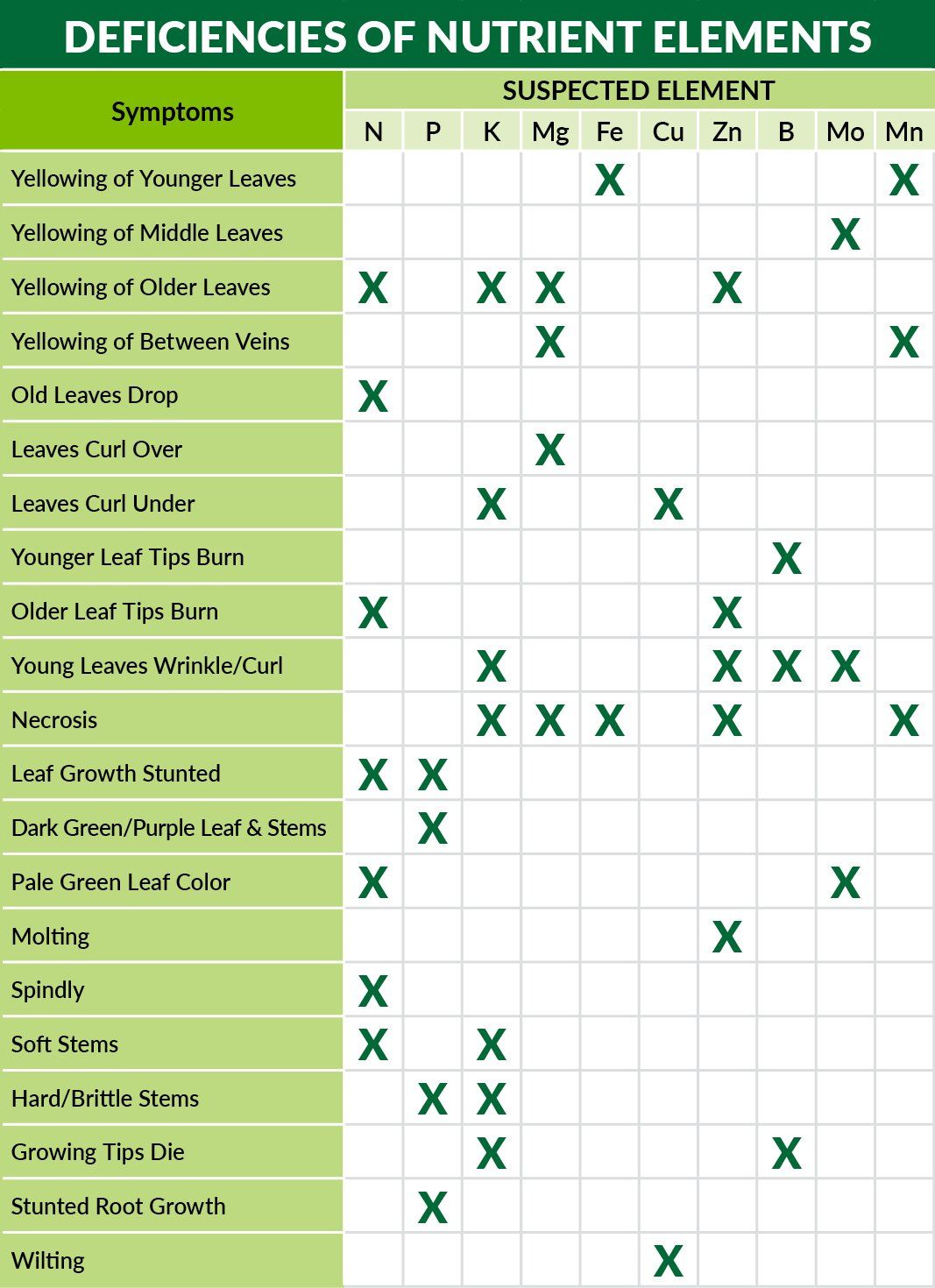
Easily the most recognizable micronutrient, iron is commonly associated with giving turf a ‘deep green up.’ Iron is involved in the synthesis of chlorophyll (dark green pigmented molecules that drive photosynthesis in plants), so it’s no surprise that plants deficient in iron suffer from a condition called “chlorosis” (yellowing sheathes). Iron is also used to transport oxygen and carry electrons (a form of energy) throughout the plant.
Manganese (Mn) –
Also involved in the synthesis of chlorophyll, manganese plays an important role in nitrogen metabolism. Manganese is also a cofactor for over 35 enzymes in different metabolic pathways. Without it, plants would struggle to produce lignin, a phenolic compound that gives plants structural rigidity and protects against biotic stresses (insect feeding and fungal pathogens).
Zinc (Zn) –
This micronutrient is important for the formation of many proteins and enzymes. Zinc deficiencies often result in poorly developed root systems, but also stunted growth and extra sensitivity to high levels of light and heat. Even when present in adequate concentrations in the soil, excessive phosphorus (either native P in the soil, or applied P fertilizers) can lead to zinc deficiencies in plants. Zinc also affects the plant’s ability to take up water from the soil. With so many biological systems dependent on zinc, a zinc deficiency could manifest itself in the form of various symptoms.
Copper (Cu) –
Many of the metabolic roles performed by copper are identical to those of zinc. Additionally, copper has a greater role in carbohydrate and protein metabolism. Copper availability is also adversely affected by increasing concentrations of phosphorous and potassium.
Boron (B) –
This little discussed micronutrient plays and important role in sugar transport as well as cell wall strength. With the window between boron deficiency and excessiveness in plants being very narrow, plants are completely dependent on uniform distribution of boron in the soil. Boron is usually taken up in the form of boric acid and is transported though xylem (the plant’s vascular system for transporting water). Ideal concentrations of boron also positively influence phosphorus and potassium uptake. Plants deficient in boron usually exhibit stunted growth.
Molybdenum (Mo) –
This element has a very specific role in plants - it assists with the conversion of nitrate to nitrite, and then nitrite into ammonia. Ammonia is then subsequently converted into amino acids by plants which are used to make proteins and other macromolecules. Molybdenum deficiencies are rare, but can be brought about by excessive use of nitrate fertilizers. The use of ammoniacal fertilizers (such as UREA, MU, AS, MAP, DAP) slows the depletion rate considerably. Plants deficient in molybdenum commonly exhibit a distinct chlorotic ring around the perimeter of blades and sheathes, sometimes accompanied by chlorotic venation.
Chlorine (Cl) - Often tagging along with a metal ion in the form of a salt, chloride (Cl-) aids in osmosis (the diffusion of water though plant cell membranes), as well as ion channel regulation. Excess chloride becomes very toxic, even at low concentrations. Chloride deficiencies are practically unheard of since salts (NaCl) are usually in abundance in most soils worldwide.
Nickel (N) –
Originally thought to have no role in plant nutrition, it is now understood that Nickel plays a critical role in Nitrogen metabolism. Nickel ions are part of the enzyme structure of urease, the enzyme that converts urea into ammonia. Even though Nickel is needed in very low concentrations, without it plants could not metabolize urea, the most abundant form of nitrogen fertilizer. Plant biologists are currently in the process of discovering how Nickel assists with disease tolerance in plants. Plants deficient in nickel show virtually no symptoms since it is almost impossible to have no nickel present in most soils.
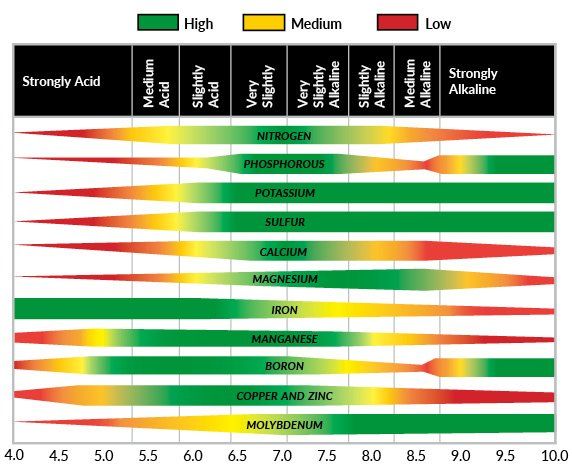
MAKING THE CONNECTION – PH AFFECTS PLANT MICRONUTRIENT BIOAVAILABILITY
Now that we have a better understanding of the roles micronutrients play in plant health, it is important to understand how soil pH can radically affect their bioavailability to plants. Bioavailability is a term used to describe elements that are both present in the soil AND in a chemical form where they are ready to be taken into the plant for nutritional use. For example, phosphorous is abundant in many soils, however much of the phosphorus is tightly bound up by metal oxides (AlO, FeO, CaO, etc.). Certain chemical bonds between phosphorous and metal oxides are so tight that those phosphorous molecules cannot be used by the plant, therefore they are “biounavailable.”
JARGON
Chlorotic Venation:
just means that all the veins look white/yellowish, but the rest of the leaf looks green. Chlorosis is a generalized term used to describe plant tissue that is not green and healthy due to nutritional deficiencies. Most of the time Chlorosis is a result of plants not being able to make chlorophyll (the dark green pigment).
Necrosis:
the death of most or all of the cells in an organ or tissue due to disease, injury, or failure of the blood supply.
Molting:
Shedding of Plant Parts focused on the anatomical, physiological, and ecological features of shedding of vegetative and reproductive parts of plants.
Spindly:
A type of growth that occurs when plants shade each other, restricting the amount of light each receives, and because seedlings compete for nutrients, growing poorly and developing thin stems and small leaves.
For professional fertilizers, humic and AMP-XC™ enriched products available, please visit TurfCare’s online Product Catalog.
For green industry professionals or others interested in ordering Turfcare products, please contact our Customer Service
to find a distributor near you. References:
http://soils.wisc.edu/facstaff/barak/soilscience326/macronut.htm
http://eldoradochemical.com/fertiliz1.htm
http://www.cropnutrition.com/manganese-in-crop-production
http://www.journalrepository.org/media/journals/AJEA_2/2013/Mar/1363870284-Hafeez322012AJEA2746.pdf
http://www.pthorticulture.com/en/training-center/role-of-copper-in-plant-culture/
http://www.pthorticulture.com/en/training-center/role-of-molybdenum-in-plant-culture/
http://www.hortidaily.com/article/13401/Role-of-nickel-in-plant-culture
https://www.gardeningknowhow.com/garden-how-to/soil-fertilizers/sulfur-in-plants.html