The TURF[TECH]REPORT – Iron’s Function in Plant Health

- DTPA iron: Largest molecules of the iron chelates. Stable up to a pH of 7.0, can bond with multiple metals and other compounds to help transport iron into the plant easier.
- EDDHA iron: Mid-range molecule size of the iron chelates. Most stable of the iron chelates; most resistant to converting into a less available form of iron at almost every pH range.
- EDTA iron: Smallest molecules of the iron chelates. Stable up to a pH of 6.5; can bond with multiple metals and other compounds to help iron transport into the plant easier. Will more readily release its iron atom once transported to its destination within the plant.
- Note: mg/kg is equivalent to parts per million (ppm)
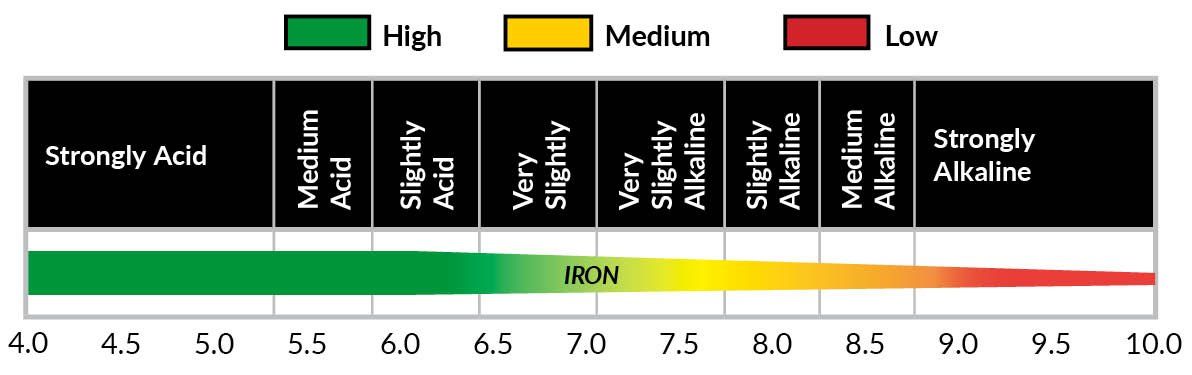
SOIL pH IMBALANCES Iron becomes readily available in a soil where pH is 6.0. Liming acidic soils is a necessity in order to optimize turf health but an overload of applications can cause iron deficiencies.
LOW LEVELS OF ORGANIC MATTER
Many plants are unable to take up nutrients from the soil without the assistance of organic compounds produced by beneficial microbes. These small living organisms consume organic matter (dead plant residues) and through digestion, turn this matter into nutrients a plant can absorb, thus fostering the kind of environment that promotes plant growth and health. Low levels of beneficial soil microbes may indirectly lead to low levels of available iron for plant consumption.
NUTRIENT IMBALANCE
The proper distribution of primary (N, P, K), secondary nutrients (Ca, Mg, S) and micronutrients (Fe, Mn, Zn, Cu, B, Mo, Cl, N) in the soil (and ultimately in the plant) is fundamental in order to fully reap the benefits of proper turf management practices ( fertilizer , control products and soil amendment applications). A balance between all these nutrients is essential in order to maintain a soil conducive to optimal turf health. For example, excessive phosphorous (P) levels can trigger iron deficiencies. Focusing on feeding the soil can enhance plant/lawn health and nutrient uptake.
Iron deficiencies in turf are sometimes difficult to pin-point because multiple factors (including but not limited to other nutrient deficiencies or imbalances) can overshadow them. The absence of iron may also be mistaken for pest infestations, disease or herbicide damage. If chlorosis-like symptoms occur, test the soil to establish existing soil fertility, pH, and composition.
MANAGING IRON DEFICIENCIES
Iron deficiencies can be managed with a short-term application of an iron-based foliar spray or with nitrogen fertilizers, although the best mode of action is prevention. New roots and root hairs become active during the iron uptake process. When outside factors interfere with root development (such as poorly balanced soils or imbalanced nutrients), iron uptake is disrupted. Identifying the true cause of an iron deficiency with a soil test can create a better understanding of why iron is depleted in turf while preventing the problem from occurring again. Focusing on (1) feeding the soil and (2) maintaining a healthy and active root system are paramount in helping to keep turf free from any potential iron deficiencies.
For professional fertilizers, humic and AMP-XC™ enriched products available, please visit TurfCare’s online Product Catalog.
For green industry professionals or others interested in ordering Turfcare products, please contact our Customer Service to find a distributor near you.
The TURFReport Highlights:
Additional Articles and Insights
















